We are probably all familiar with the spectrum of solar light. Its most spectacular natural manifestation is probably the rainbow. Small rain droplets from a distant storm illuminated by sunlight separate the solar light into its spectral components and reveal a range of red, orange, yellow, green, blue, indigo and violet colours.
The spectrum of solar light is continuous under everyday circumstances. Photons generated by the thermonuclear reactions fuelling the interior of our star, after an arduous journey, reach the surface and are emitted out over a wide range of frequencies (a continuum).
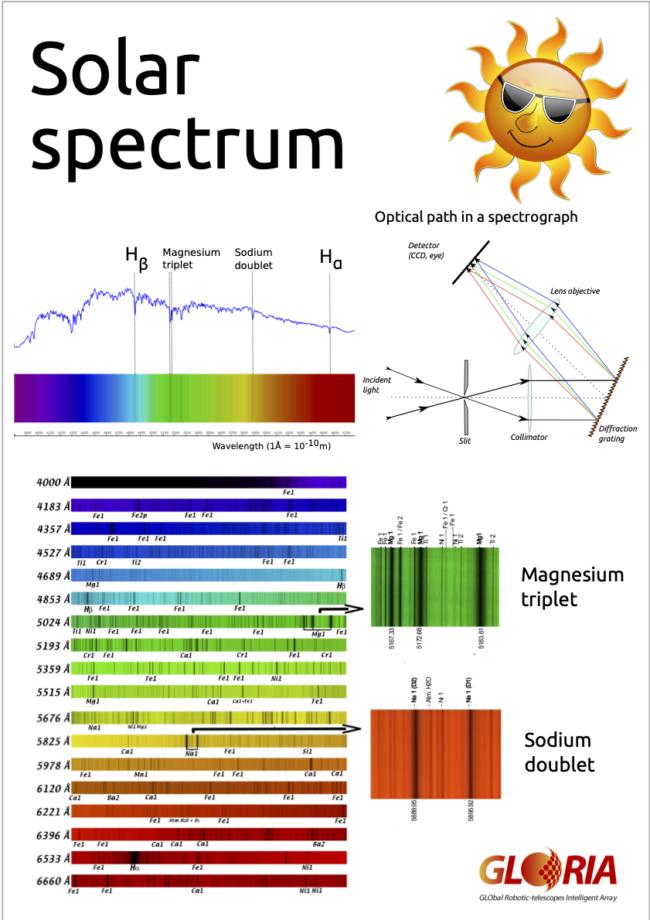
While travelling through the solar atmosphere, photons at very specific wavelengths are absorbed by the elements present in those regions. This process leads to the appearance of dark lines superposed to the solar continuum. The main elements responsible for these absorption lines are Hydrogen, Magnesium, Sodium, Calcium and Iron. There is a host of other contributors, too, both neutral elements and ions, as the following diagram shows.
During a total solar eclipse, when the Moon is about to entirely occult the Sun’s disc (the photosphere), the usual solar spectrum rapidly evolves into what is usually called the “flash spectrum”. The photospheric continuum vanishes through the bottom of the lunar valleys and it is replaced by what is mostly a discrete spectrum with bright and faint lines.
The following sequence of images shows the photospheric continuum fading away and the flash spectrum coming into view.

The flash spectrum is an emission spectrum, mainly due to light coming from the lowermost layers of the solar atmosphere. The bright reddish arc on the right and the acqua and blue arcs on the left are emitted by Hydrogen. The main yellow arc is due to Helium. The bright green arcs are emitted by Magnesium and the fainter yellow arcs next to the intense Helium emission are due to Sodium. The signature of a host of fainter lines coming from a variety of elements in neutral or ion for is also present.
The continuum photospheric spectrum is ordinarily orders of magnitude more intense than the flash spectrum. But during total solar eclipses, at the onset of totality, the Moon performs its magic, occulting, even if barely, the Sun’s disc and letting the flash spectrum shine in all its glory. Besides making for pretty colourful images, the flash spectrum gives us a very detailed view of the interface between photosphere and solar atmosphere and it can be used, among other things, to estimate the value of the solar radius.
